
(v) In some cases, elements with similar properties have been placed in different groups. But there is no similarity among the elements in the two sub-groups of a particular group.
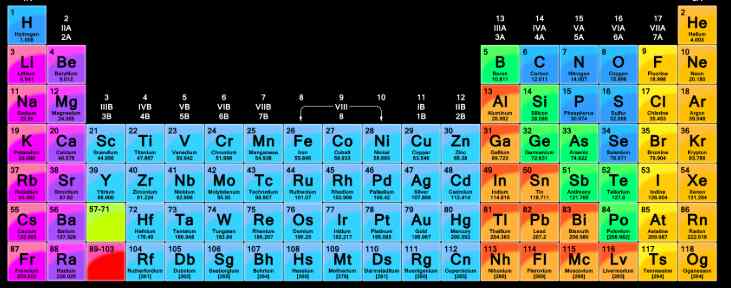
(iv) According to Mendeleev, the elements placed in the same group must resemble in their properties. Since, periodic table has been framed on the basis of increasing atomic masses of the elements, different positions must have been allotted to all the isotopes of a particular element. (iii) We know that the isotopes of an element have different atomic masses but same atomic number. (ii) Although the elements in the Mendeleev’s periodic table have been arranged in order of their atomic masses, but in some cases the element with higher atomic mass precedes the element with lower atomic mass. Thus, its position is the Mendeleev’s periodic table is controversial. But it also resembles halogens of group VII A in many properties. (i) Hydrogen has been placed in group IA along with alkali metals. (iii) Mendeleev corrected the atomic masses of certain elements with the help of their expected positions and properties. (ii) This helped to a great extent in the discovery of these elements at a later stage. (i) This made the study of the elements quite systematic in the sense that if the properties of one element in a particular group are known, those of others can be pridicted. (iii) There are seven periods (numbered from 1 to 7) or, horizontal rows in the Mendeleev’s periodic table. The zero group contains elements belonging to inert gases or noble gases and elements present have zero valency. Group VIII consists of nine elements which are arranged in three triads. (ii) There are nine groups indicated by Roman Numerals as I, II, III, IV, V, VI, VII, VIII and zero. (i) In the periodic table, the elements are arranged in vertical rows called groups and horizontal rows known as periods. The horizontalĭescription of Mendeleev’s Periodic Table Mendeleev arranged the elements known at that time in order of increasing atomic massesĪnd this arrangement was called periodic table.Įlements with similar characteristics were present in vertical rows called groups. Mendeleev’s Periodic Law: The physical and chemical properties of the elements are a periodic (ii) When noble gas elements were discovered at a later stage, their inclusion in the table disturbed the entire arrangement. After that, every eighth element did not possess the same properties as the element lying above it in the same group. (i) This classification was successful only up to the element calcium. Newlands called it law of octaves because similar relationship exists in the musical notes also. John Newlands proposed the law of octaves by stating that when elements are arranged in order of increasing atomic masses, every eighth element has properties similar to the first. The triads given by Dobereiner were helpful in grouping some elements with similar characteristics together, but he could not arrange all the elements known at that time into triads. In 1829, Dobereiner arranged certain elements with similar properties in groups of three in such a way that the atomic mass of the middle element was nearly the same as the average atomic masses of the first and the third elements.


#Periodic table chemistry class pdf


 0 kommentar(er)
0 kommentar(er)
